BioEthanol Production
Process modeling and simulation with ProSimPlus
|
|
|
Process modeling
The feedstock used in this process is corn stover. The main compounds are (on dry basis, wt.) cellulose (37.4%), hemicellulose (21.1%) and lignin (18.0%). The modeling starts just after the washing of the feedstock which induces an increase of the feedstock moisture.
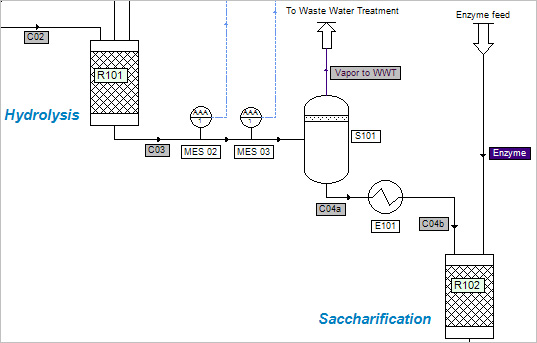 A flash separation (S101) at atmospheric pressure is present between the hydrolysis reactor and the saccharification reactor to evacuate part of the water and some by-products. Another flash (S102) is used after the fermentation reactor (R103) to separate out the incondensable CO2.
A flash separation (S101) at atmospheric pressure is present between the hydrolysis reactor and the saccharification reactor to evacuate part of the water and some by-products. Another flash (S102) is used after the fermentation reactor (R103) to separate out the incondensable CO2.
Three reactions are represented in the reactors:
| | 1. |
Hydrolysis reactor (R101): heating the feedstock to 190°C at high pressure (12.1 atm) with an acid catalyst (H2SO4). Most of the hemicellulose is converted to xylose. |
| | 2. | Saccharification reactor (R102): an enzymatic reaction occurs, which converts most of the cellulose to glucose. |
| | 3. | Fermentation reactor (R103): most of the glucose and the xylose are converted to ethanol and carbon dioxide. |
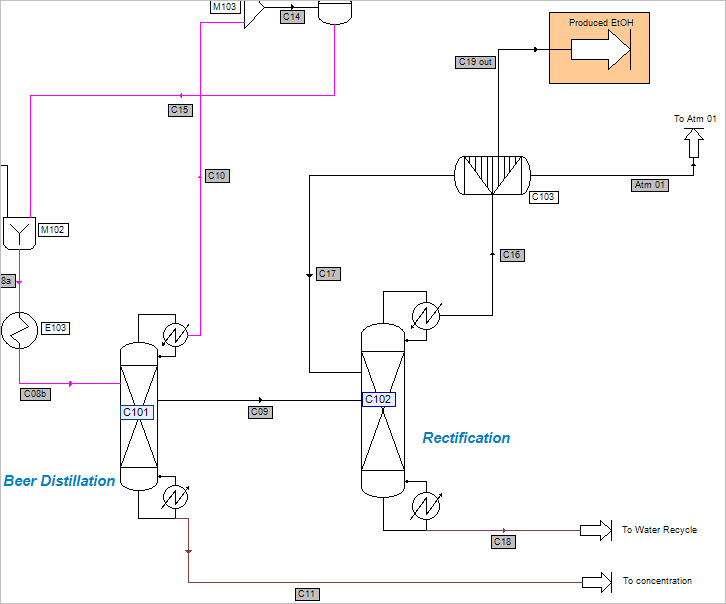 The first distillation column (C101) separates out the remaining incondensable CO2, which is recovered at the vapor distillate (stream C10). The water, sugars and the un-reacted part of the feedstock are produced at the bottom
(stream C11) and the ethanol is withdrawn at a side-stream (stream C09). This side-stream goes to a second distillation column (C102).
The ethanol, is recovered at the vapor distillate (stream C16).
The first distillation column (C101) separates out the remaining incondensable CO2, which is recovered at the vapor distillate (stream C10). The water, sugars and the un-reacted part of the feedstock are produced at the bottom
(stream C11) and the ethanol is withdrawn at a side-stream (stream C09). This side-stream goes to a second distillation column (C102).
The ethanol, is recovered at the vapor distillate (stream C16).
At the bottom, water is recovered and recycled in the process (stream C18). The ethanol is dried in a molecular sieve adsorption unit (C103). This part of the process is modeled by a component splitter. A scrubber (C104) separates ethanol and water contained in the gas stream coming from the flash separator (S102) after the fermentation reactor and the vapor distillate of the first distillation column (C101).
Components
The components used in this example are listed in the following table.
| Name | Chemical formula | Use in the process |
| Acetate | C2H4O2 | Acetate groups present in the hemicellulose polymer |
| Acetic acid | C2H4O2 | Coming from acetate hydrolysis and fermentation by-product |
| Carbon dioxide | CO2 | Fermentation product |
| Cellobiose | C12H22O11 | Coming from cellulose hydrolysis and saccharification |
| Cellulose | C5H10O5 | Feedstock |
| Corn steep liquor | Unknown | Bacteria feed nitrogen source |
| Diammonium phosphate (DAP) | (NH4)2HPO4 | Bacteria feed nitrogen source |
| Enzyme | CH1.57N0.29O0.31S0.007 | Saccharification enzyme |
| Ethanol | C2H6O | Desired product |
| Furfural | C5H4O2 | Hemicellulose hydrolysis by-product |
| Glucose | C6H12O6 | Coming from cellulose hydrolysis and saccharification |
| Glycerol | C3H8O3 | Fermentation by-product |
| Hemicellulose | C5H8O4 | Feedstock |
| Lactic acid | C3H6O3 | Fermentation by-product |
| Lignin | C10H13.9O1.3 | Feedstock |
| Oxygen | O2 | Fermentation product |
| Succinic acid | C4H6O4 | Fermentation by-product |
| Sulfuric acid | H2SO4 | Acid catalyst |
| Water | H2O | Product moisture, washing and reaction product |
| Xylitol | C5H12O5 | Fermentation by-product |
| Xylose | C5H10O5 | Coming from hydrolysis and saccharification |
| Z. mobilis | CH1.8O0.5N0.2 | Fermentation bacteria |
|
Some of these compounds are not included in the ProSim standard database provided with ProSimPlus. They have been represented with similar components of which some properties have been modified as detailed in the following table.
| Component | Properties |
| Acetate | Modeled as Acetic acid. |
| Cellobiose | Modeled as Glucose. Modification: molecular weight(E) |
| Cellulose | Modeled as Glucose. Modification: molecular weight(E), ideal gas heat of formation(E), liquid molar volume(E), liquid heat capacity(E) |
| CSL | Modeled as Water. Modification: glucose vapor pressure |
| Enzyme | Modeled as Glucose. |
| Hemicellulose | Modeled as Glucose. Modification: molecular weight(E), ideal gas heat of formation(E), liquid molar volume(E), liquid heat capacity(E) |
| Lignin | Modeled as Glucose. Modification: molecular weight(E), liquid molar volume(E), liquid heat capacity(E) |
| Xylitol | Modeled as Hemicellulose. Modification: molecular weight(E) |
| Xylose | Modeled as Glucose. Modification: molecular weight(E), critical temperature(E), critical pressure(E), critical volume(E), acentric factor(E), ideal gas heat of formation(E), liquid molar volume(E), liquid heat capacity(E) |
| Z. mobilis | Modeled as Glucose. Modification: ideal gas heat of formation(E), liquid molar volume(E), liquid heat capacity(E) |
Thermodynamic model
This system contains polar compounds (like water and ethanol), which induce strong interactions in the liquid phase.
The working pressure being weak (from 1 to 5 atm), the vapor phase behavior can be consider similar to that of an ideal gas. Consequently the NRTL model has been selected. The required binary interaction parameters that
were not available in ProSimPlus database were estimated with a predictive model, using the dedicated functionality offered by the thermodynamic module of ProSimPlus.
|
ADDITIONAL INFORMATION
Contact ProSim by e-mail (sales @ prosim.net) or through the contact page from ProSim website (click on the button):

|
|
|
|
|
|